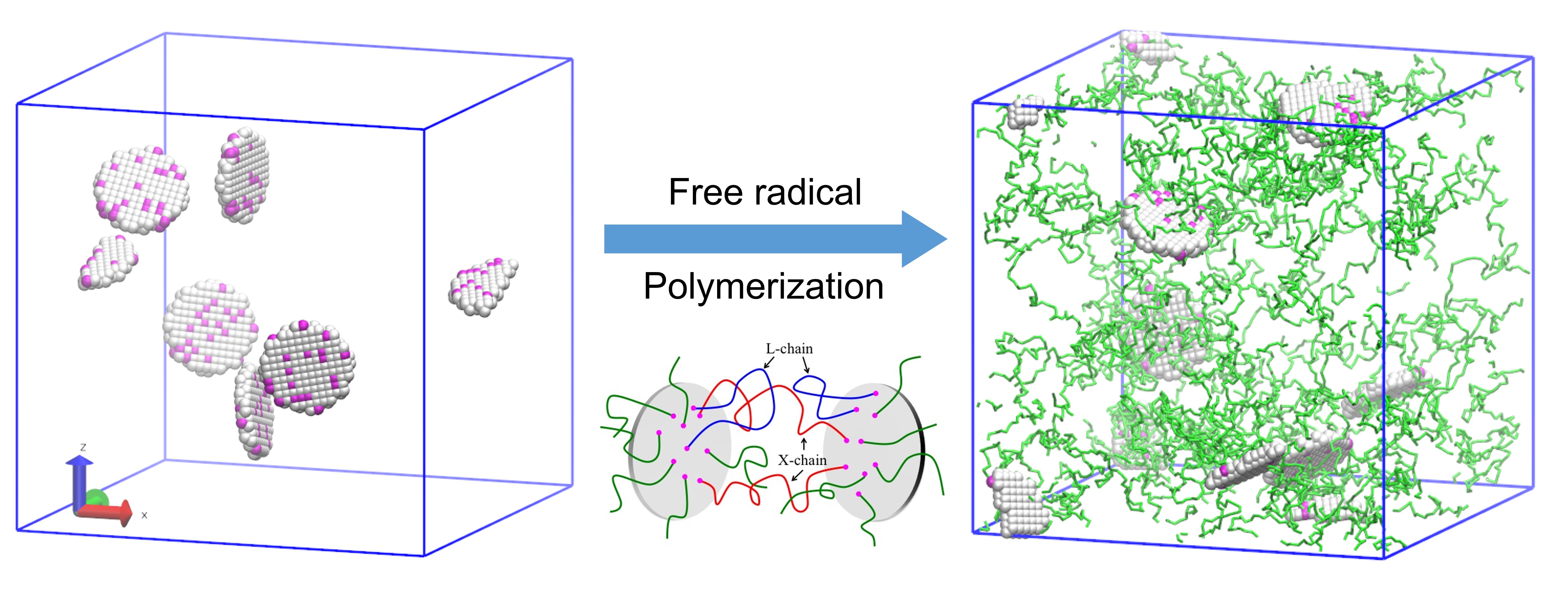
Modeling Free Radical Polymerization Using Dissipative Particle Dynamics
Understanding the details of free radical polymerization (FRP) in multi-component mixtures and solutions is of great importance for the synthesis of polymeric functional materials. Using the framework of dissipative particle dynamics (DPD), we develop a new computational approach to model FRP that couples the reactions kinetics for the polymerization processes to the dynamics of the complex fluid. We specifically consider two mechanisms of chain termination: disproportionation and combination. We analyze the effects of initiation, propagation, and termination on the polymerization kinetics in three-dimensional bulk polymerization by varying the corresponding reaction probabilities. Our model not only allows us to capture the interplay between hydrodynamics and reaction kinetics, but also provides an effective means to model polymerization in the presence of solid inclusions. We demonstrate the latter feature by simulating the formation of polymer-clay nanocomposite gels by FRP in solution in the absence of organic cross-linking agents, where the exfoliated clay particles serve as multi-functional cross-linkers for the polymer network. We observe that increasing the volume fraction of clay particles can lead to an increase in the number of inter-particle cross-linking chains, which could improve the mechanical properties of the material. Our findings provide insight into the polymerization kinetics of the FRP, as well as potential guidelines for tailoring experimental conditions to achieve the desired polymerization products.
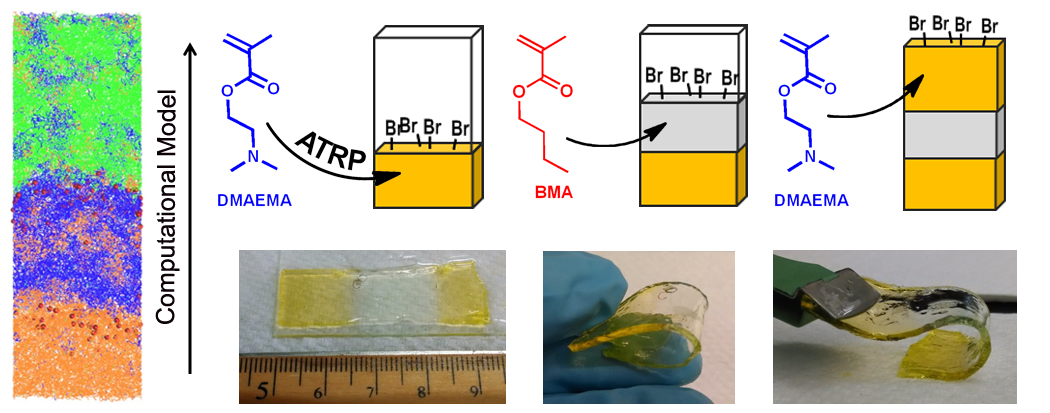
Designing Stackable, Covalently-Fused Gels Using ATRP
Combining modeling and experiment, we created multilayered gels where each layer was "stacked" on top of the other and covalently interconnected to form mechanically robust materials, which could integrate the properties of the individual layers. In this process, a solution of new initiator, monomer, and cross-linkers was introduced on top of the first gel, and these new components then underwent living (co)polymerization to form the subsequent layer. We simulated this process using dissipative particle dynamics (DPD) to isolate factors that affect the formation and binding of chemically identical gel as well as incompatible layers. Analysis indicates that the covalent bond formation between the different layers is primarily due to reactive chain-ends, rather than residual cross-linkers. In the complementary experiments, we synthesized multilayered gels using either free radical (FRP) or atom transfer radical polymerizations (ATRP) methods. Polymerization results demonstrated that chemically identical materials preserved their structural integrity independent of the polymerization method. For gels encompassing incompatible layers, the contribution of reactive chain-ends plays a particularly important role in the integrity of the material, as indicated by the more mechanically robust systems prepared by ATRP. These studies point to a new approach for combining chemically distinct components into one coherent, multifunctional material as well as an effective method for repairing severed gels.
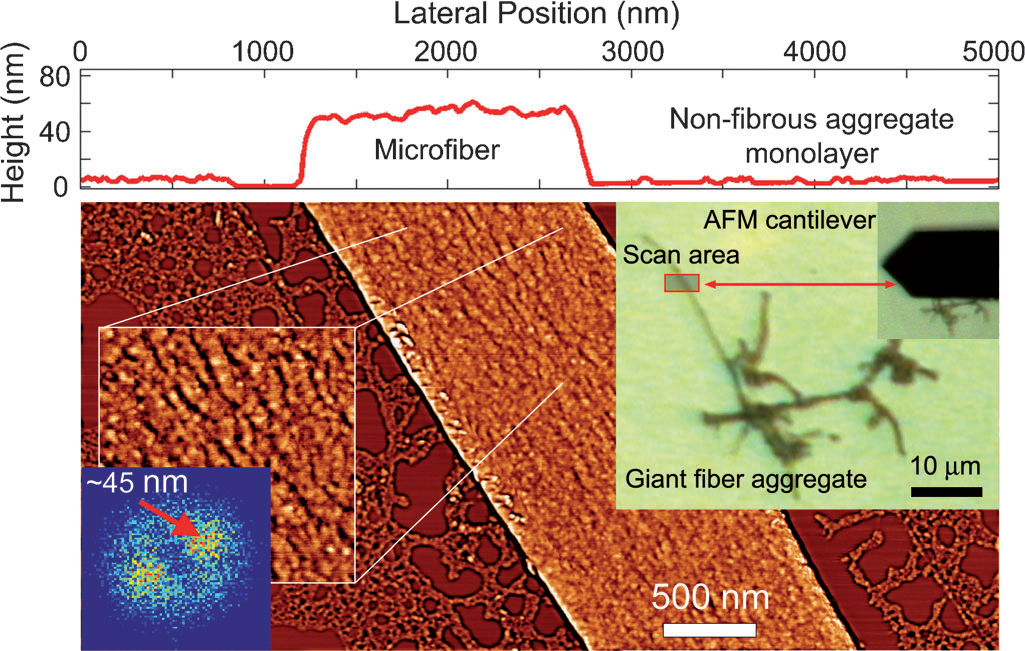
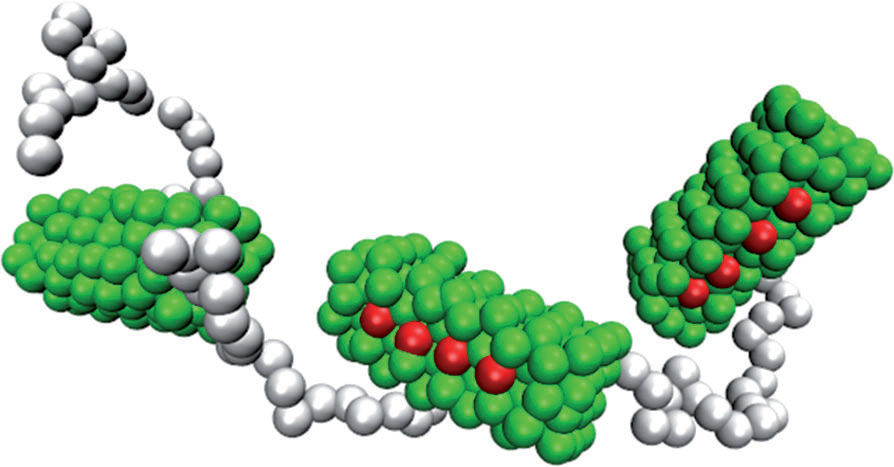
Designing Giant Nanofibers in Bioconjugate Polymer System
We demonstrate a simple bioconjugate polymer system that undergoes reversible self-assembling into extended fibrous structures, reminiscent of those observed in living systems. It is comprised of green fluorescent protein (GFP) molecules linked into linear oligomeric strands through click step growth polymerization with dialkyne poly(ethylene oxide) (PEO). Confocal microscopy, atomic force microscopy, and dynamic light scattering revealed that such strands form high persistence length fibers, with lengths reaching tens of micrometers, and uniform, sub-100 nm widths. We ascribe this remarkable and robust form of self-assembly to the cooperativity arising from the known tendency of GFP molecules to dimerize through localized hydrophobic patches and from their covalent pre-linking with flexible PEO. Dissipative particle dynamics simulations of a coarse-grained model of the system revealed its tendency to form elongated fibrous aggregates, suggesting the general nature of this mode of self-assembly.
 |
 |
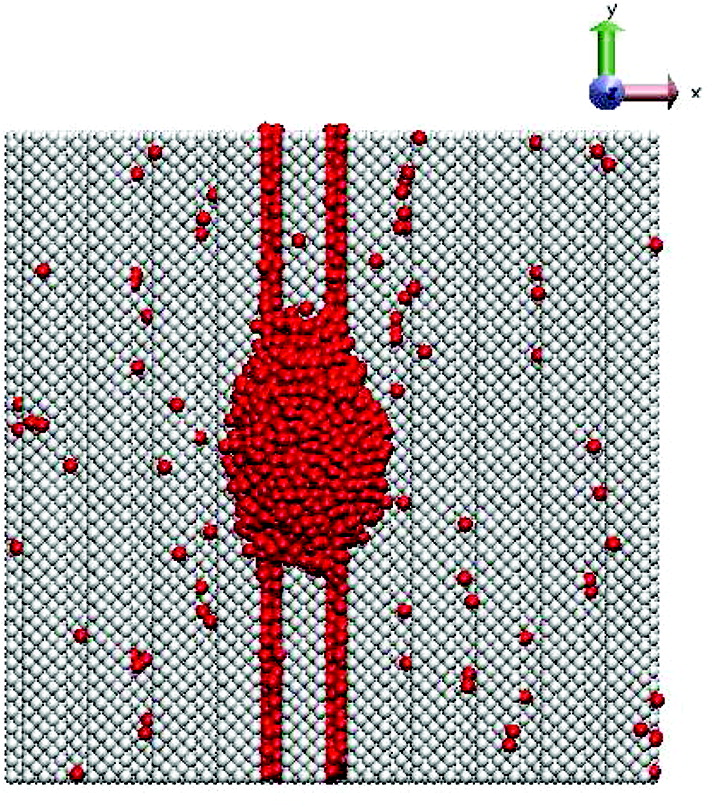
Designing Regenerative Polymer Gel
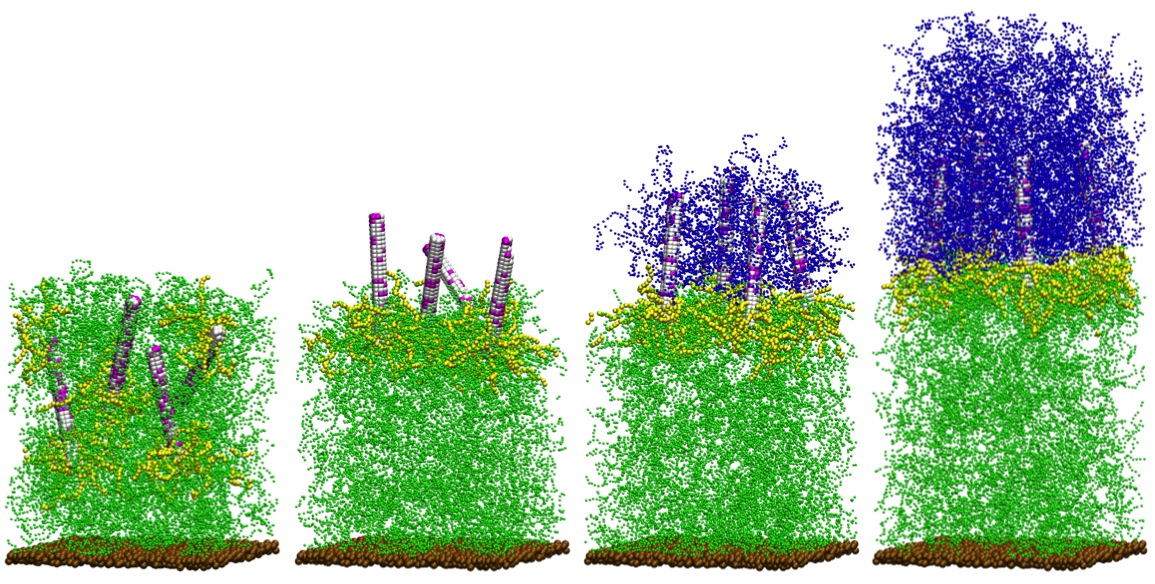
An elusive goal in materials science is designing systems that mimic the remarkable ability of amphibians to regrow limbs. While self-healing materials can mend local defects, there are virtually no examples of materials that can regenerate themselves. The advent of such regenerative materials could dramatically extend the useful lifetime of manufactured products. Through new computational models, we design a nanorod-filled gel that effectively regenerates the gel matrix when a layer of the material is sliced-off. With this layer removed, the nanorods diffuse to the newly formed interface and extend into the outer solution, which contains monomers and a small fraction of cross-linkers. Polymerization initiated from the surfaces of rods leads to chains that become cross-linked to form a new gel that resembles the severed layer. After the initial cut, the regeneration requires no external intervention; synergistic interactions among all components in this system enable the vital processes leading to regrowth, which could be repeated with subsequent cuts.
In biology, tissue regeneration is guided by an internalized "instruction set", that involves signaling molecules that mediate the vital processes in the regrowth: initiation, propagation, and termination. Analogous to biological systems, fully synthetic self-regenerating materials should incorporate the following elements. First, the system must encompass a component that not only senses the removal of material but also initiates the regrowth. Second, the system must involve a means of continuing or propagating the desired growth. Finally, when the material reaches a certain size, there must be a means of terminating the ongoing reactions. By developing new computational models, we design a self-regenerating material that undergoes these processes to produce a remarkable form of regrowth when a significant portion of the system is removed.
 |
 |
Harnessing Vesicles as Janus Particles Carrier
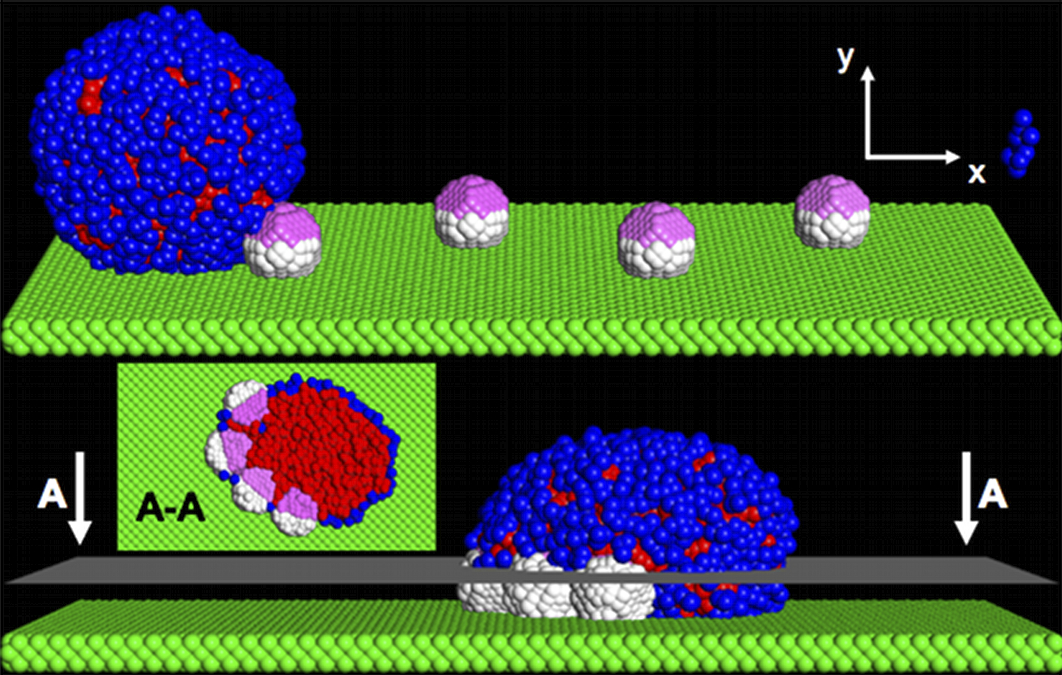
Using dissipative particle dynamics (DPD) simulations, we model the interaction between nanoscopic lipid vesicles and Janus nanoparticles in the presence of an imposed flow. Both the vesicle and Janus nanoparticles are localized on a hydrophilic substrate and immersed in a hydrophilic solution. The fluid-driven vesicle successfully picks up Janus particles on the substrate and transports these particles as cargo along the surface. The vesicle can carry up to four particles as its payload. Hence, the vesicles can acts as nanoscopic "vacuum cleaners", collecting nanoscopic debris localized on the floors of the fluidic devices. Importantly, these studies reveal how an imposed flow can facilitate the incorporation of nanoparticles into nanoscale vesicles. With the introduction of a hydrophobic domain on the substrate, the vesicles can also robustly drop off and deposit the particles on the surface. The controlled pickup and delivery of nanoparticles via lipid vesicles can play an important step in the bottom-up assembly of these nanoparticles within small-scale fluidic devices.
Slip Boundary Condition
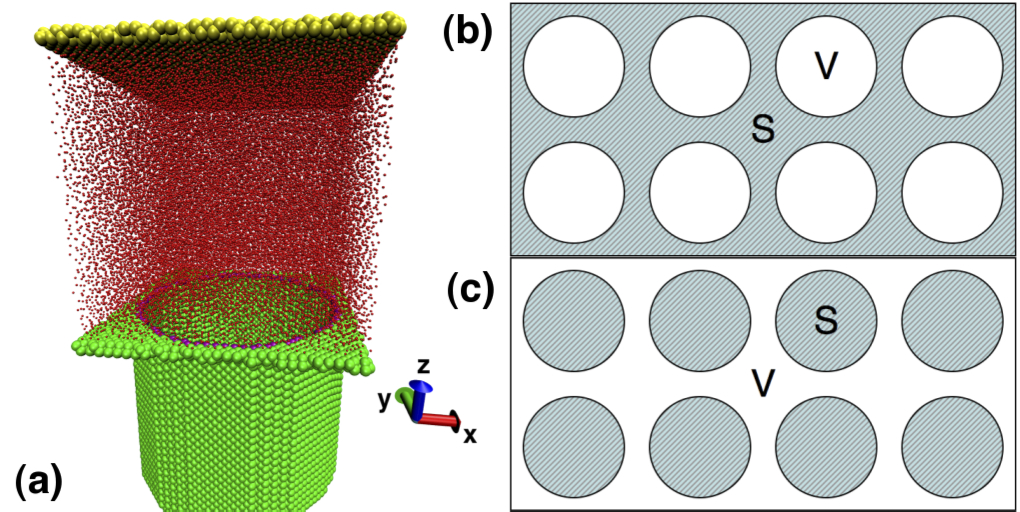
The flow boundary condition at fluid-solid interfaces has been a subject haunting the fluid dynamics community for hundreds of years. Until recent decades, the boundary condition has merely been an assumption based on the observations at macroscale due to the lack of precise measuring techniques at the interface. The most common assumption of the hydrodynamic velocity boundary condition made at continuum scale is the no-slip condition, i.e. all three components of the fluid velocity at the immediate vicinity of the solid surface are equal to the respective velocity components of the surface. The inquiry of slip between fluid and solid has been under meticulous investigation for decades. Recent controlled experiments have demonstrated apparent violations of the no-slip condition. Intense research on slip was fostered by the prosperous applications in micro- and nanofluidics, and enormous practical applications rely on understanding the nature of slip. For example, the permeability of channels and porous materials is tremendously reduced as the channel size decreases to micrometer or nanometer scale under no-slip condition. To bypass the stringent tradeoff between flow rate and channel size, and achieve good permeability for nanochannels, allowing the fluid to slip against the solid is crucial. Revealing the underlying physics of slip and further manipulating slip at nanoscale can profoundly impact the future of single molecule analysis, nanotribology, energy conversion and desalination.
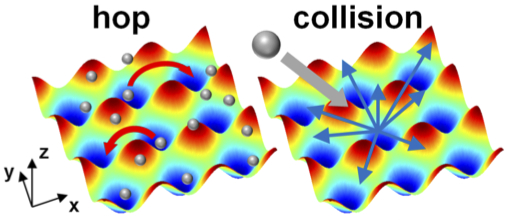
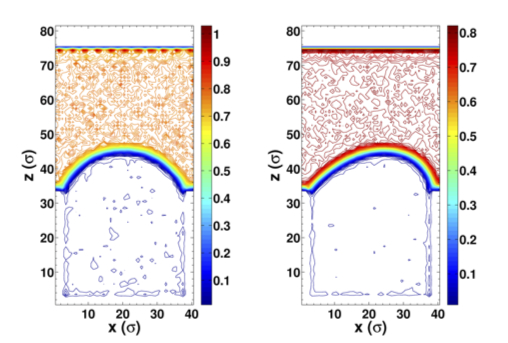
Our study explores the interfacial friction mechanisms and their interplay with the nanoscale slip behavior using non-equilibrium molecular dynamics simulations. Our results show that there is an abrupt jump of slip length at a critical shear rate, corresponding to the transition from "defect slip" at low shear rates to "collective slip" at high shear rates. Here, we identified two mechanisms of interfacial friction: surface potential and collision mechanisms. Their impacts on slip are elaborated through a quantitative scaling estimation and our results show that both mechanisms contribute to the defect slip at low shear rates, while the collision mechanism dominates the collective slip at high shear rates. We also verify the importance of the bulk viscous heating via a comparison among different thermostat strategies.
We also study the slip behavior of the simple fluid on nanoengineered superhydrophobic surfaces. The surfaces with cylindrical nanoholes and nanopillars are investigated to represent the difference in the continuity of liquid-vapor interface. By successfully generating entrapped bubble on the surface with nanoholes, we obtain the effect of the meniscus curvature quantified by the protrusion angle on the effective slip length. Our slip behaviors at nanoscale are in good agreement with the previous macro- or microscale numerical simulations, which verifies the possibility of fine tuning the boundary condition on nanoengineered surfaces by controlling the shape of the meniscus. By eliminating the meniscus effect, the limiting behaviors of the effective slip length when the gas fraction changes are studied on the surfaces with nanoholes and nanopillars with flat liquid-vapor interface. The asymptotic behaviors show quantitative agreement with the macroscopic scaling laws, which demonstrates the feasibility to characterize the behaviors of nanosystems with some well-established continuum concepts. Moreover, we elucidate that for nanobubble, the effective slip has no apparent shear-rate dependence contrast to the microscopic behavior, because the subtle change in the bubble volume and small motion of contact line has to be considered when their scales are comparable to the scale of the system. We hope that this work could provide guidance of designing low friction nanoengineered surfaces and tailoring their slip properties, and further benefit the macroscale drag reduction.
 |
 |
Droplet Wetting
The fascinating features and properties of interfacial hydrodynamics of interacting liquids and solids inspired numerous designs of material surfaces that can capture and facilitate desired functions. The well-known "lotus effect" that denotes roughness-induced superhydrophobicity and self-cleaning abilities of biological and artificial surfaces has been adopted in various applications in many disciplines. A notable application is designing self-cleaning mechanisms that can detect and remove hazard particles as the liquid droplet rolls away. With increasing interests in miniaturized designs of nano- and microfluidic systems and devices, scientists are able to obtain desired wetting properties for specific applications that do not operate at the macroscale. Therefore, the theory of wetting needs investigation at small scales, because the liquid may be more sensitive to the surface structure and chemistry.
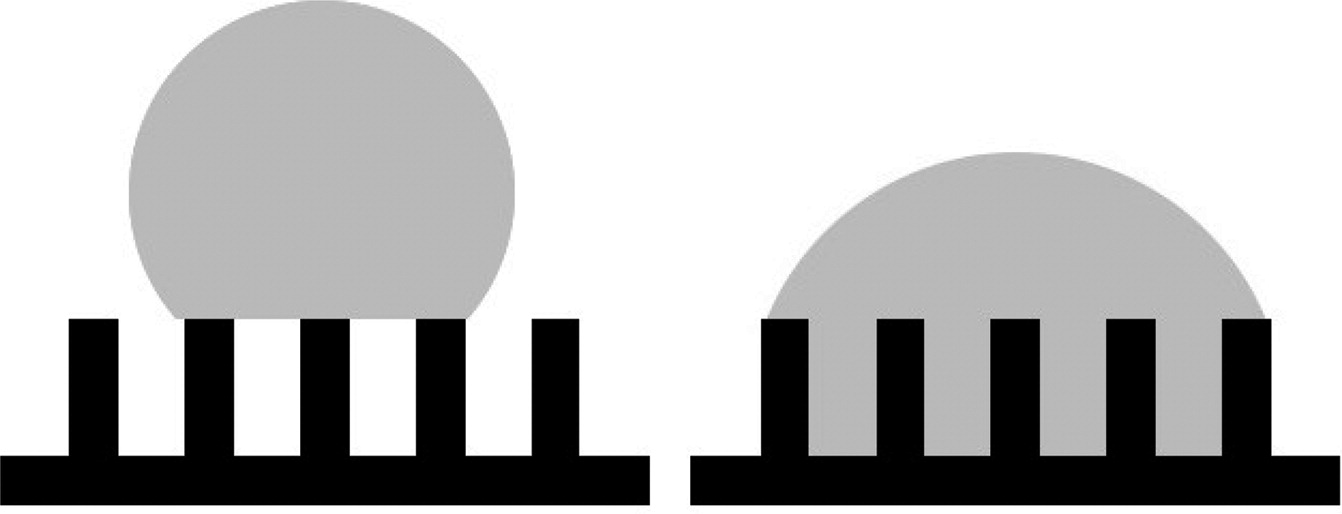
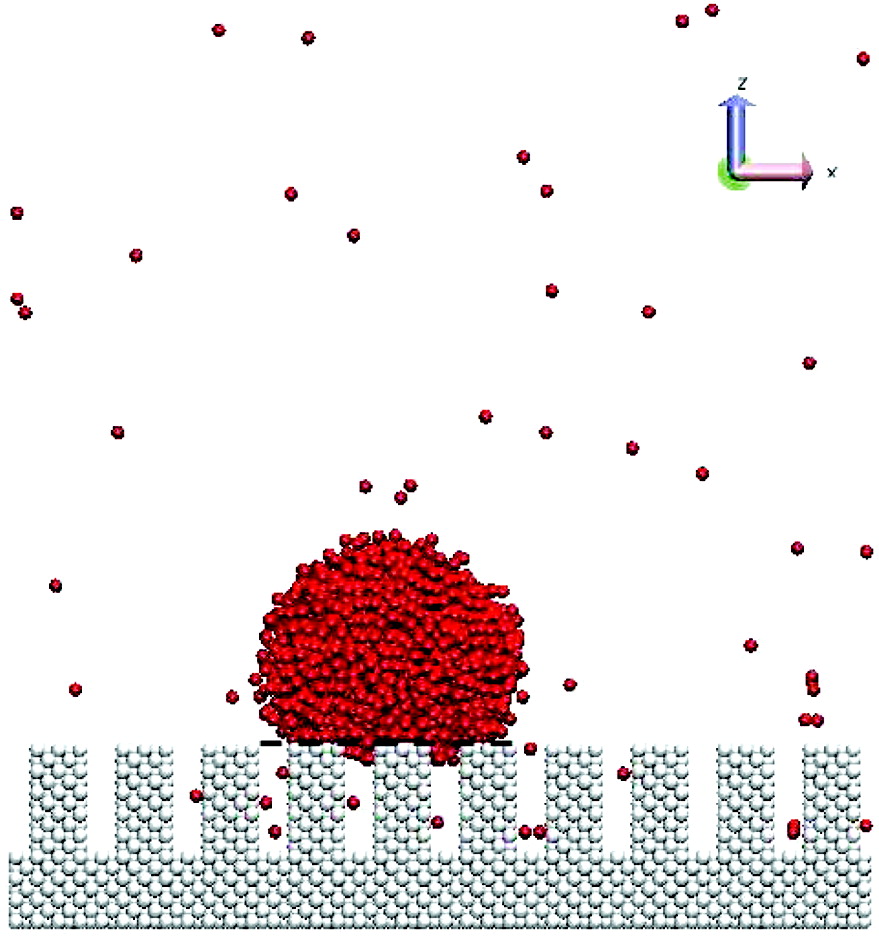
Nanoscale wetting on groove-patterned surfaces is thoroughly studied using molecular dynamics simulations. The results are compared with Wenzel's and Cassie's predictions to determine whether these continuum theories are still valid at the nanoscale for both hydrophobic and hydrophilic types of surfaces when the droplet size is comparable to the groove size. The wetting properties are determined by measuring contact angles of the liquid droplet at equilibrium states. Correlations are established between the contact angle, roughness factor r, and surface fraction f. The results show that, for hydrophobic surfaces, the contact angle as a function of roughness factor and surface fraction on nanogrooved surfaces obeys the predictions from Wenzel's theory for wetted contacts and Cassie's theory for composite contacts. However, slight deviations occur in composite contacts when a small amount of liquid penetration is observed. The contact angle of this partial wetting cannot be accurately predicted using either Cassie's or Wenzel's theories. For hydrophilic surfaces, only wetted contacts are observed. In most cases, the resulting contact angles are found to be higher than Wenzel's predictions. At the nanoscale, high surface edge density plays an important role, which results in contact line pinning near plateau edges. For both hydrophobic and hydrophilic surfaces, substantial amount of anistropic spreading is found in the direction that is parallel to the grooves, especially at wetted or partially wetted contacts.
 |
 |

