Currently Our group has four projects:
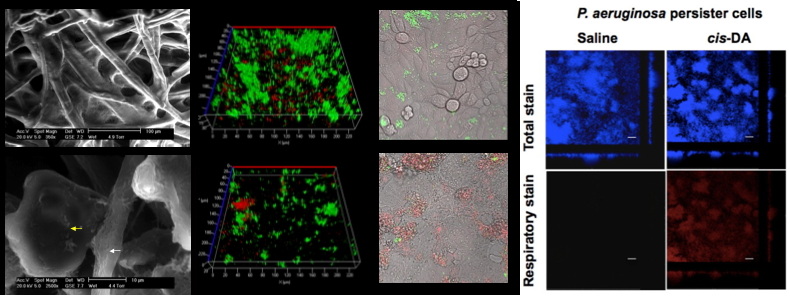
Persister cells are a sub-population of cells within the general bacterial population considered to be in a dormant/low metabolic state, and therefore tolerant to antimicrobial treatments. These cells are not resistant to antimicrobials, but merely unresponsive to them. It is generally thought that persister cells are not recognized by the immune system and that they are partially responsible for the recurrence of infection. Currently we have 2 projects related to persister cells a. Phosphate reservoir and awakening of Pseudomonas aeruginosa persister cells. We are determining whether the effect of cis-DA on persister cells is due to the modulation of the phosphate reservoir by monitoring the overall phosphate pool present within bacterial cells during persister cell formation and awakening both in P. aeruginosa WT and in various mutant strains impaired in polyphosphate generation and degradation. b. Host interaction with persister cells. We are determining whether the immune system recognizes persister cells and whether awakening these cells will lead to an earlier recognition and engulfment of these cells.
2. The modeling and elucidation of the interaction between single and dual species biofilms of S. aureus and P. aeruginosa biofilms with their surrounding environment.
Presently there is limited knowledge regarding the interactions of different bacterial species present within multispecies biofilms and as biofilms found in the environment are composed, mostly, of several bacterial species this is a promising upcoming area of research. Recent evidence in our laboratory showed that in dual-species biofilms composed by P. aeruginosa and S. aureus, the species composition ratios within the microcolonies is altered throughout biofilm development. Currently, we are evaluating the relative expression of several S. aureus and P. aeruginosa quorum sensing genes known to play a role in biofilm development, and determining the distribution of fluorescent labelled P. aeruginosa and S. aureus, within the dual-species biofilms, when co-cultured on plastic and when co-infecting human bronchial epithelial cells. This is an interdisciplinary project with Dr. Guy German and Dr. Scott A. Craver.
3. Effect of dysbiosis, increase of Staphylococcus aureus, and lipid loss on the onset of atopic dermatitis. (AD) is a chronic disease in which the skin commonly exhibits inflammation and exudative lesions together with a decrease of the Stratum Corneum (SC) lipids and increased populations of bacteria. The largest increase of bacterial population between healthy and AD skin occurs with S. aureus. This population change is believed to be caused by the depletion of sphingosine, a potent antimicrobial. We have found that on ex vivo samples with delipidated SC S. aureus biofilms are present at higher concentrations and are localized in the topographical canyon regions. While, in control samples, the bacterial growth is heterogeneous and avoids the topographical canyon regions. Currently we are examining the influence of barrier disruption caused by lipid depletion on the ability of S. aureus biofilms to infect and populate the SC surface and we are determining the influence of lipid content on bacterial growth and localization within healthy skin and comparing it to delipidized (mimics AD) skin. In addition, we are also mimicking healthy and AD conditions on primary epithelial keratinocytes and evaluating the growth of S. aureus biofilms and gene expression during the biofilm development. This is an interdisciplinary project with Dr. Guy German.
4. Engineering of a gut microbiome.
The goal of this project is to determine if and how ingested metal oxide nanoparticles alter microorganism populations and intestinal function. Currently we are developing a "mock" community of bacteria found in the human gastrointestinal (GI) tract. The mock community is composed by Lactobacillus rhamnosus, Bifidobacterium bifidum, Streptococcus salivarius, and Enterococcus faecalis. We have found that the presence of L. rhamnosus is required for B. bifidum to grow as a biofilm community and that when culturing the four bacterial species a steady state is reached at day 5. Furthermore, we have also found that introduction of single bacterial species to the GI tract model improved GI function. This is an interdisciplinary project with Dr. Gretchen Mahler.